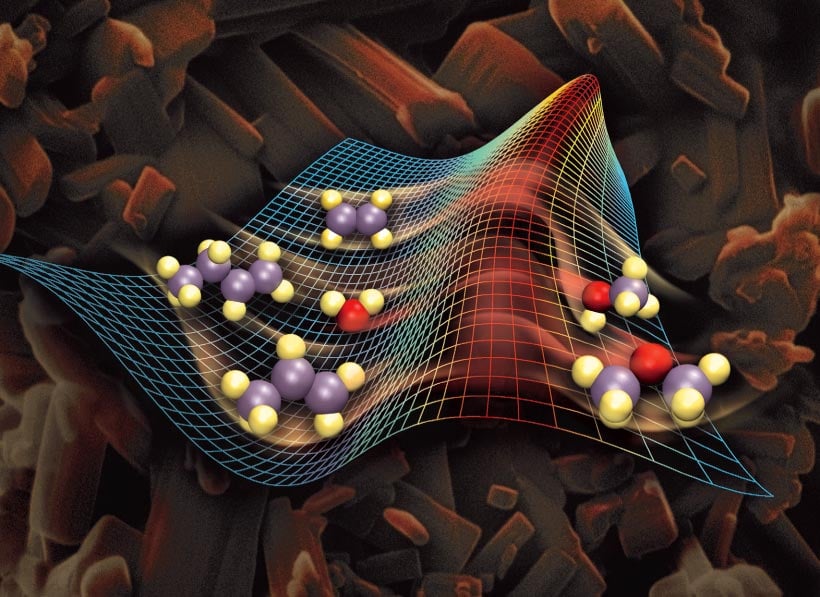
Function Of Catalysts In Chemical Reactions . With a helping hand from a catalyst, molecules that might take years to. — a catalyst changes the activation energy, e a, of a reaction by providing an alternate pathway for the reaction. the function of a catalyst is only to alter the speed of the reaction, already occurring at a particular rate. A catalyst does not alter. — explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; — a catalyst is some material that speeds up chemical reactions. — a catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more. List examples of catalysis in. — catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed.
from scitechdaily.com
the function of a catalyst is only to alter the speed of the reaction, already occurring at a particular rate. — catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. — a catalyst changes the activation energy, e a, of a reaction by providing an alternate pathway for the reaction. — explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; — a catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more. List examples of catalysis in. — a catalyst is some material that speeds up chemical reactions. A catalyst does not alter. With a helping hand from a catalyst, molecules that might take years to.
Science Made Simple What Are Catalysts?
Function Of Catalysts In Chemical Reactions — a catalyst is some material that speeds up chemical reactions. — a catalyst changes the activation energy, e a, of a reaction by providing an alternate pathway for the reaction. With a helping hand from a catalyst, molecules that might take years to. — catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. — a catalyst is some material that speeds up chemical reactions. the function of a catalyst is only to alter the speed of the reaction, already occurring at a particular rate. List examples of catalysis in. — explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; A catalyst does not alter. — a catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free download ID6634764 Function Of Catalysts In Chemical Reactions A catalyst does not alter. — a catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more. — explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; List examples of catalysis in. — a catalyst is some material that speeds up. Function Of Catalysts In Chemical Reactions.
From www.dreamstime.com
Catalyst Surface with Catalytic Reaction Stock Vector Illustration of bind, matter 218305638 Function Of Catalysts In Chemical Reactions — a catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more. — catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. With a helping hand from a catalyst, molecules that might take years to. — a catalyst changes the. Function Of Catalysts In Chemical Reactions.
From www.slideserve.com
PPT Basic Chemistry Concepts PowerPoint Presentation, free download ID5923586 Function Of Catalysts In Chemical Reactions — explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; the function of a catalyst is only to alter the speed of the reaction, already occurring at a particular rate. — catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. With a helping hand. Function Of Catalysts In Chemical Reactions.
From www.alamy.com
Chemical Reactions of catalyst and product Stock Vector Image & Art Alamy Function Of Catalysts In Chemical Reactions A catalyst does not alter. the function of a catalyst is only to alter the speed of the reaction, already occurring at a particular rate. With a helping hand from a catalyst, molecules that might take years to. — catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. — explain. Function Of Catalysts In Chemical Reactions.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation ID1803655 Function Of Catalysts In Chemical Reactions — a catalyst is some material that speeds up chemical reactions. A catalyst does not alter. — a catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more. — catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. —. Function Of Catalysts In Chemical Reactions.
From jackwestin.com
Rate Processes Catalysts Rate Processes In Chemical Reactions And Equilibrium MCAT Function Of Catalysts In Chemical Reactions — a catalyst changes the activation energy, e a, of a reaction by providing an alternate pathway for the reaction. List examples of catalysis in. the function of a catalyst is only to alter the speed of the reaction, already occurring at a particular rate. With a helping hand from a catalyst, molecules that might take years to.. Function Of Catalysts In Chemical Reactions.
From courses.lumenlearning.com
12.7 Catalysis Chemistry Function Of Catalysts In Chemical Reactions — a catalyst is some material that speeds up chemical reactions. List examples of catalysis in. — a catalyst changes the activation energy, e a, of a reaction by providing an alternate pathway for the reaction. A catalyst does not alter. — catalyst, in chemistry, any substance that increases the rate of a reaction without itself being. Function Of Catalysts In Chemical Reactions.
From www.youtube.com
Catalytic Converter Working Principle 2 way and 3 way, Function of catalyst [Animation Video Function Of Catalysts In Chemical Reactions — a catalyst changes the activation energy, e a, of a reaction by providing an alternate pathway for the reaction. List examples of catalysis in. With a helping hand from a catalyst, molecules that might take years to. — explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; — a catalyst. Function Of Catalysts In Chemical Reactions.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID5967535 Function Of Catalysts In Chemical Reactions — a catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more. List examples of catalysis in. With a helping hand from a catalyst, molecules that might take years to. — explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; —. Function Of Catalysts In Chemical Reactions.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube Function Of Catalysts In Chemical Reactions List examples of catalysis in. — a catalyst changes the activation energy, e a, of a reaction by providing an alternate pathway for the reaction. — a catalyst is some material that speeds up chemical reactions. — explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; With a helping hand from. Function Of Catalysts In Chemical Reactions.
From www.ck12.org
Catalysts Example 1 ( Video ) Chemistry CK12 Foundation Function Of Catalysts In Chemical Reactions A catalyst does not alter. — a catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more. — a catalyst is some material that speeds up chemical reactions. List examples of catalysis in. the function of a catalyst is only to alter the speed of the reaction,. Function Of Catalysts In Chemical Reactions.
From wou.edu
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry Function Of Catalysts In Chemical Reactions With a helping hand from a catalyst, molecules that might take years to. A catalyst does not alter. — a catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more. — a catalyst changes the activation energy, e a, of a reaction by providing an alternate pathway for. Function Of Catalysts In Chemical Reactions.
From wou.edu
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry Function Of Catalysts In Chemical Reactions — a catalyst is some material that speeds up chemical reactions. A catalyst does not alter. List examples of catalysis in. — explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; — a catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the. Function Of Catalysts In Chemical Reactions.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst. Download Scientific Function Of Catalysts In Chemical Reactions — catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. — a catalyst is some material that speeds up chemical reactions. — explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; List examples of catalysis in. — a catalyst binds to a reactant. Function Of Catalysts In Chemical Reactions.
From scitechdaily.com
Science Made Simple What Are Catalysts? Function Of Catalysts In Chemical Reactions A catalyst does not alter. List examples of catalysis in. With a helping hand from a catalyst, molecules that might take years to. the function of a catalyst is only to alter the speed of the reaction, already occurring at a particular rate. — a catalyst binds to a reactant and it increases the number of collision between. Function Of Catalysts In Chemical Reactions.
From www.slideserve.com
PPT Starter 1)Definition of catalysts 2) Difference between homogeneous and heterogeneous Function Of Catalysts In Chemical Reactions — a catalyst is some material that speeds up chemical reactions. A catalyst does not alter. List examples of catalysis in. — a catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more. With a helping hand from a catalyst, molecules that might take years to. —. Function Of Catalysts In Chemical Reactions.
From exorbcrky.blob.core.windows.net
Catalyst Meaning Chem at Rick Franks blog Function Of Catalysts In Chemical Reactions — a catalyst changes the activation energy, e a, of a reaction by providing an alternate pathway for the reaction. A catalyst does not alter. — explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; — catalyst, in chemistry, any substance that increases the rate of a reaction without itself being. Function Of Catalysts In Chemical Reactions.
From www.slideserve.com
PPT Enzymes Biological Catalysts PowerPoint Presentation, free download ID5736986 Function Of Catalysts In Chemical Reactions With a helping hand from a catalyst, molecules that might take years to. — a catalyst changes the activation energy, e a, of a reaction by providing an alternate pathway for the reaction. A catalyst does not alter. the function of a catalyst is only to alter the speed of the reaction, already occurring at a particular rate.. Function Of Catalysts In Chemical Reactions.